Introduction
Cholesterol is an extremely important sterol in the tissues of animals. It is an organic molecule and a type of lipid. It is a fat-like substance, which is waxy in texture, and found in all the cells of our body, as it is essential for carrying out many functions such as synthesis of hormones, synthesis of vitamin D and also acts as an integral structural component of the membranes of animal cells. Cholesterol can exist as free cholesterol or as a storage form, in which it is combined with a long chain fatty acid (eg: cholesteryl ester). Although cholesterol is essential, excess of it can cause some serious problems in the body like heart attack, hyperthyroidism, diabetes mellitus, etc.
Sources of cholesterol
Cholesterol present in the body is derived from dietary foods, hydrolysis of cholesteryl esters and synthesis of cholesterol (more than half of the cholesterol is present due to synthesis).
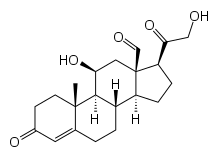
Figure: Chemical structure of cholesterol
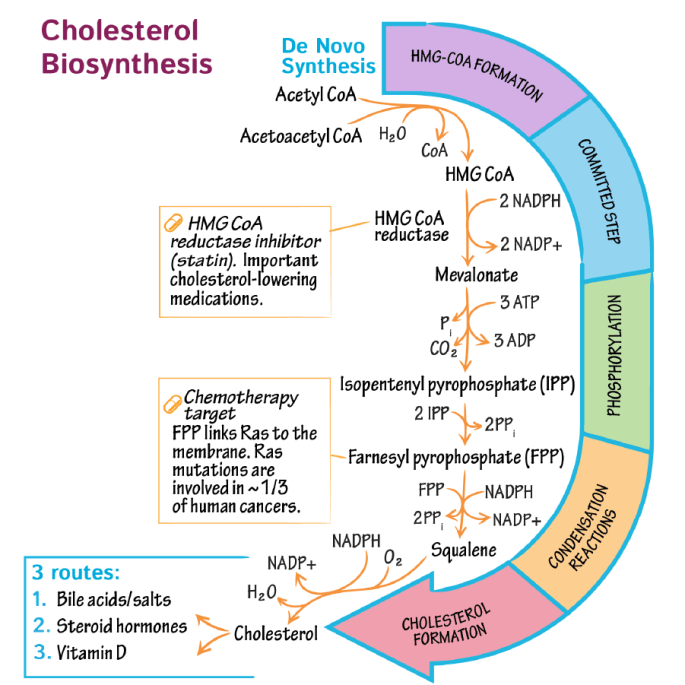
Cholesterol Biosynthesis
The biosynthesis of cholesterol takes place in the endoplasmic reticulum and the cytosol of the cell. Majorly, the process takes place in nucleated cells of the liver, like hepatic cells. The biosynthesis takes place in 5 major steps:
- Biosynthesis of mevalonate:
- Mevalonate, a conjugate base of mevalonic acid, is formed from acetyl CoA during the synthesis of cholesterol.
- This takes place in the cytosol of the cell.
- Aceto-acetyl CoA is formed by the condensation of 2 molecules of Acetyl CoA, catalyzed by thiolase.
- This product further condenses with another acetyl CoA molecule to give HMG-CoA, a reaction catalysed by HMG-CoA synthase.
- This HMG-CoA is reduced to mevalonate by NADPH. This is catalysed by HMG-CoA reductase.
- This is an important regulatory step as well in the biosynthesis of cholesterol, where HMG-CoA reductase plays a key role.
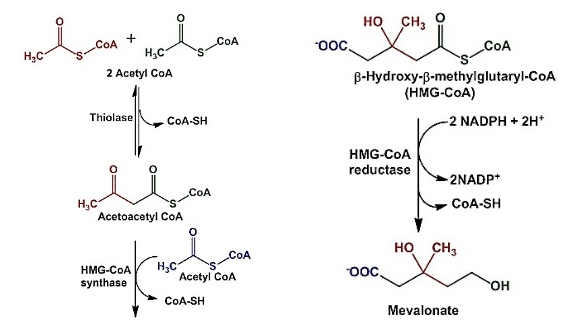
Figure: Biosynthesis of Mevalonate
- Formation of isoprenoid units
- Isoprenoid units are formed when mevalonate is phosphorylated sequentially in the presence of ATP and Mg2+ and 3 kinases to give mevalonate-3-phospho-5-diphosphate.
- The final kinase of the phosphorylation, mevalonate-3-phospho-5-diphosphate undergoes decarboxylation to give an active isoprenoid unit.

Figure: Biosynthesis of isoprenoid
- Formation of Squalene
- Six molecules of Isopentenyl Pyrophosphate (C5) undergo condensation to form a 30 carbon molecule known as Squalene.
- It is a sequential pathways which proceeds as: C5——>C10——->C15——>C30
- Intermediates that are observed are geranyl diphosphate (C10), farnesyl diphosphate (C15) and finally which goes on to form squalene (C30).
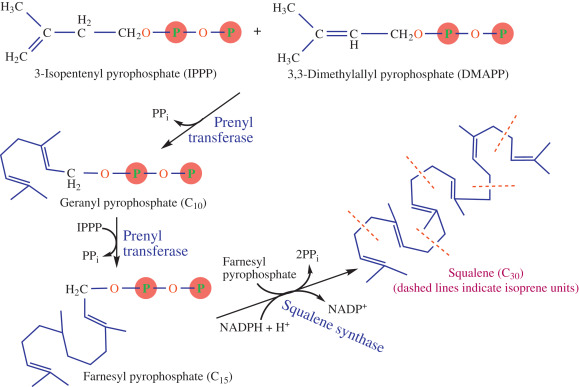
Figure: Biosynthesis of Squalene
- Formation of Lanosterol
- Squalene is converted to squalene-2,3-epoxide in the endoplasmic reticulum by squalene epoxidase.
- After this, ring closure occurs in the presence of oxidosqualene: lanosterol cyclase to give lanosterol, which is a freshly formed cyclic structure.
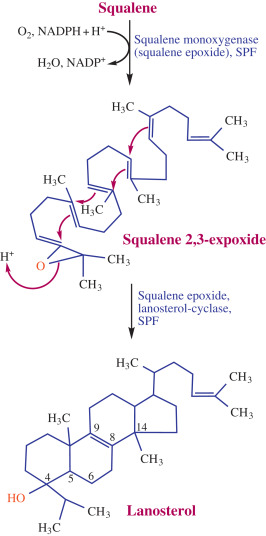
Figure: Biosynthesis of Lanosterol
- Cholesterol formation
- Finally, cholesterol is formed from lanosterol in the membranes of the endoplasmic reticulum.
- This takes place through a number of changes in the side chains and the steroid nucleus.
- An intermediate known as desmosterol is also formed due to the shift of a double bond.
- This double bond of the side chain is reduced, which ultimately forms cholesterol.

Figure: Formation if cholesterol from lanosterol
Conclusion
Cholesterol is thus synthesized by nucleated cells in the body through a long pathway involving numerous enzymes and steps. It is an essential molecule as it has various biological functions such as it acts as the precursor for steroid hormones and bile salts, it is required for nerve transmission and is a major constituent of plasma membrane and lipoproteins.

BY- Shaily Sharma (MSIWM041)