BY: Reddy Sailaja M (MSIWM030)
BLOOD
Blood is a specialized body fluid, comprises of plasma and the cells that circulate throughout the human body. It supplies oxygen, glucose, antibodies, vitamins, electrolytes, heat, hormones, and immune cells across the body for life survival. It removes carbon dioxide and other waste generated from the cells in the body.
BLOOD COMPONENTS
Blood is made up of three main components: plasma, blood cells (red and white) and platelets.
Plasma: Plasma occupies 55% of blood fluid in human beings. Plasma comprises of 92% water and the remaining 8% contains carbon dioxide, glucose, hormones, proteins etc.
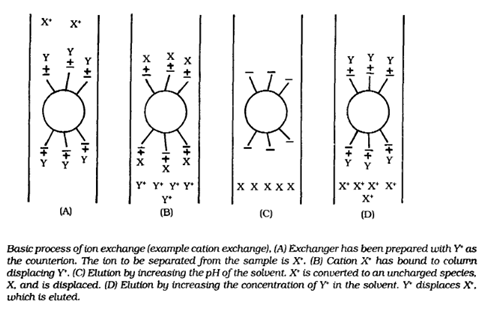
Blood cells: Blood cells comprise of 45% of total blood fluid. These are produced in bone marrow by the process called ‘hematopoiesis’ from a common precursor cell (hematopoietic stem cells). Then these blood cells mature into red blood cells (RBCs), white blood cells (WBCs) and platelets. The organs like lymph nodes, liver and spleen regulate the generation, destruction and regulation of blood cells.

Figure 1: Blood cells production by hematopoiesis
Red blood cells: RBCs are also called erythrocytes. They are double concave shaped structures without nucleus. Approximately, 4.5 – 6.2 million/microliter are present in the blood. Their main function is to carry oxygen from lungs to all parts of the body. RBCs contain a special protein called ‘Hemoglobin’ that aids in oxygen transport. RBCs have a life span of 120 days.
White blood cells: WBCs are otherwise known as leucocytes. WBCs are vital in fighting against invading pathogens and infections. Approximately 3700 – 10500/microliter are present in the blood. Apart from fighting infection, WBCs also help heal wounds by ingesting dead cells and debris, protects against foreign entity that enter blood stream and fights against cancerous cells.
The following are the types of WBCs that are produced in response to the kind of infection (bacterial vs fungal vs viral vs parasitic). Life span varies from hours to days to years depending upon the type of WBCs. WBCs are majorly divided into two subtypes: Granulocytes and agranulocytes. Granulocytes contains protein containing granules in their cytoplasm. Eosinophils, basophils and neutrophils constitute granulocytes. Monocytes and lymphocytes constitutes agranulocytes. The following table explains the types of WBCs’, their nature and function.
| Type of white blood cell | Percentage of abundance in WBCS (%) | Function |
| Basophils | 0.5 – 1 | Basophils produce in response to parasite infections, allergy and bone marrow damage. It secretes histamine – involve in allergic reactions and heparin – an anticoagulant that aids blood clotting at the site of injury and subsequent wound healing. |
| Eosinophils | 2 – 4 | Eosinophils defend against bacterial and parasite infections by releasing toxic substances and results in immflamatory reaction. |
| Neutrophils | 60 – 70 | First line of defense against invading pathogens. They attack the pathogens, engulf and digest them by phagocytosis process and maintain normal health. |
| Monocytes | 3 – 8 | Maintains tidiness of the blood and other tissues by clearing the dead pathogen particles and damaged cells and their debris. |
| Lymphocytes | 20 – 25 | B – cells produce antibodies against bacterial, viral and fungal infection. T – cells are two types, cytotoxic T-cells kills the antigens and helper T-cells aid antibody production from B-cells. Natural killer cells attack any foreign object that comes in contact with the body. |
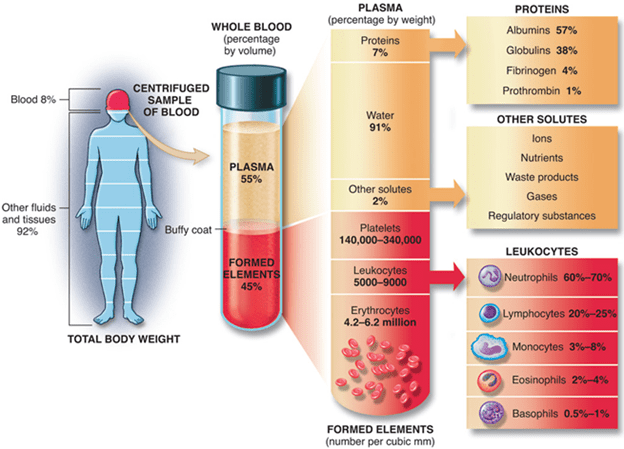
Figure 2: Blood and its components
Blood platelets: These are also called thrombocytes. Approximately 1,50,000 – 4,00,000 platelets/microliter are present in the blood. Platelets help clot the blood to stop bleeding during injury by protects the wound against further infection.
In brief, blood functions include:
- Oxygen supply to cells and tissues
- Supply essential nutrients – glucose, aminoacids, fatty acids,
- Removal of waste material – carbon dioxide, urea, lactic acid
- Fighting against infections
- Regulating body temperature and pH balance
- Transport hormones and transmits neuromessages
Blood disorders:
Blood disorders often cause life threatening situations as the infection spreads out throughout the body by blood circulation. General blood disorders are as follows:
RBCs disorder – Anemia: Low number of RBCs in blood cause anemic situation. This results in low oxygen supply in the body, fatigue and pale skin.
WBCs disorder – Cancer: Lymphoma, myeloma and leukemia are the major blood related cancers.
Platelets disorder – Internal blood clots: these clots block blood supply and can be dislodged and spread through various organs like lungs, heart, brain etc, which can be fatal to the body.