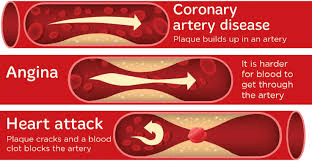
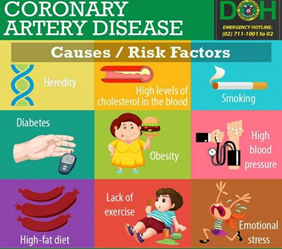
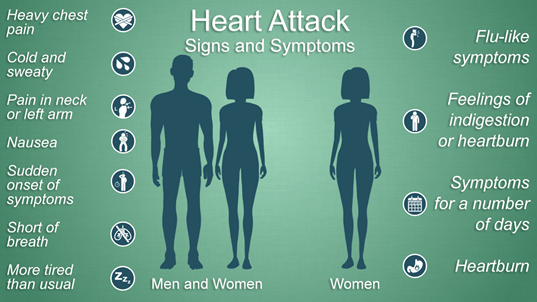
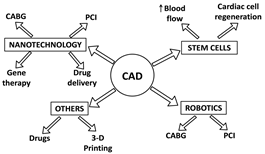
BY: SHAILY SHARMA (MSIWM041)
Chromatography, broadly, is a technique to separate two phases which are mutually immiscible. The phases are brought in contact with each other and one of these phases is stationary while the other phase is a mobile phase which moves over the surface of the stationary phase or percolates it. The interactions of the sample mixture which are established after the movement of the mobile phase over the stationary phase lead to the separation of the desired compound present in the mobile phase based on the differences in the physio-chemical properties of the two mediums.
There are various techniques used in the process of chromatography like plane chromatography, paper chromatography, thin layer chromatography, column chromatography etc.
Based on these techniques, further different types of chromatographic procedures have been curated which include adsorption chromatography, partition chromatography, gel permeation chromatography, ion exchange chromatography and affinity chromatography.
GEL PERMEATION CHROMATOGRAPHY:
Gel permeation chromatography is a chromatographic procedure that separates molecules based on their molecular size. This method has various other names such as molecular sieve, gel filtration and molecular exclusion chromatography.
This method is widely used due to its many advantages, some of which are:
- It is a very gentle technique that permits the separation of labile molecules.
- It is a technique in which the solute recovery is almost 100%.
- The reproducibility of this technique is high.
- The technique is not very time consuming and is relatively inexpensive.
PRINCIPLE:
The principle of gel permeation chromatography is relatively very simple.
A long column filled with gel beads or porous glass granules is allowed to attain equilibrium with a solvent which is suitable for the separation of the desired compound or molecule.
Assuming that the mixture to be separated contains molecules of varying sizes, when this mixture will be passed through the column containing the beads;
- The larger molecules pass through the interstitial spaces between the beads and do not enter the spaces inside the beads. This occurs because the diameter of the molecule is larger than the pore size of the beads.
- Therefore, the larger molecules are able to pass down the column with little or no resistance.
- The small molecules however, have a size which is smaller than the diameter of the pores of the beads and therefore these molecules enter the beads and reach the end of the column after a longer period of time due to the resistance that is created by the passage of the molecules through the beads.
The degree of retardation (or the extent of the time taken by the molecule to reach the bottom) is proportional to the time it spends inside the pores of the gel which is a function of the molecules pore size and the molecules size.
The molecules which have a diameter equal to or smaller than the pore size, do not enter and are said to be excluded. Therefore, the exclusion limit of a gel can be defined as the molecular weight of the smallest molecule which is not capable of entering the pores. For e.g. linear polysaccharides and fibrous proteins have a much lower exclusion limit as compared to the globular proteins of comparable molecular weight.
TYPES OF GELS USED:
A good gel filtration medium should possess a few properties or characteristics like:
- The material of the gel should be chemically inert.
- It should contain a small number of ionic groups.
- The material of the gel should have a wide variety of particle and pore sizes.
- It should have a uniform particle and pore size.
- The material of the gel should be of high mechanical rigidity.
Some examples of the types of gels used in gel permeation chromatography include sephadex, polyacrylamide, agarose, styragel etc.
TECHNIQUE/PROCESS OF GEL PERMEATION CHROMATOGRAPHY:
This chromatographic technique can be performed wither by column or by thin layer chromatography techniques.
The initial step of the process is:
- Preparation of the beads:
Prior to use, the gels used in the column must be converted to their swollen form by either soaking them in water or using a weak salt solution. The greater is the porosity of the gel beads, more will be the time taken for them to attain equilibrium and reach their maximum size.
If porous glass granules are used, the gel beads need not be hydrated at all.
- Preparation of the column:
The gel beds, in their swollen form, are mounted or supported on a column on a glass wool plug or nylon net and the previously swollen gel is added in the form of slurry. The preparation is then allowed to settle. Air bubbles must be removed by connecting the column to a vacuum pump. It must be noted that the level of the liquid must not go lower than the top of the bed.
- Addition of the sample:
The sample must be added from the top of the column using a funnel and the volume of the sample that must be added depends on the size and the type of the gel that is used in the preparation of the stationary phase.
- Collection of the sample:
The eluant used is steadily added and the effluent can be collected in various various fractions in separate tubes.
- Detection of sample:
The most common detection methods include the collection and analysis of the fraction and continuous methods in which the UV absorption, refractive index or radioactivity is measured.
APPLICATIONS:
- Gel permeation chromatography is mainly and widely used for the separation of biomolecules and purification. Biomolecules like proteins, hormones, enzymes etc. can be separated using this technique.
- This technique is especially useful for the separation of 4S and 5S tRNA.
- This technique is used for the separation of salts and small molecules from macromolecules.
- Gel permeation chromatography is also chiefly used for the detection of molecular weights of macromolecules.
Sources:
Biophysical Chemistry Principles and Techniques by Updhyay and Nath